
Chemistry, 30.03.2021 15:50, jfitness11
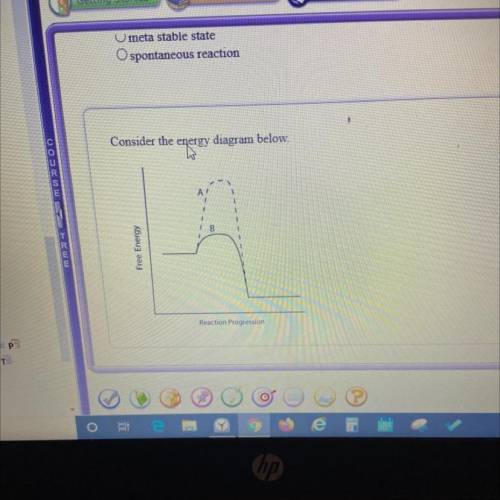
Which line indicates a higher reaction rate?
A. A because it has a lower activation energy.
B. B because it has a lower activation energy.
C. A because its Arxn is much lower.
D. B because its AGеxn is much lower.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:30, ethanmel21
Which compound contains both ionic and covalent bonds? a) hbr b)cbr4 c)nabr d) naoh
Answers: 2

Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1
Do you know the correct answer?
Which line indicates a higher reaction rate?
A. A because it has a lower activation energy.
Questions in other subjects:



Biology, 18.03.2020 18:09




Mathematics, 18.03.2020 18:10









