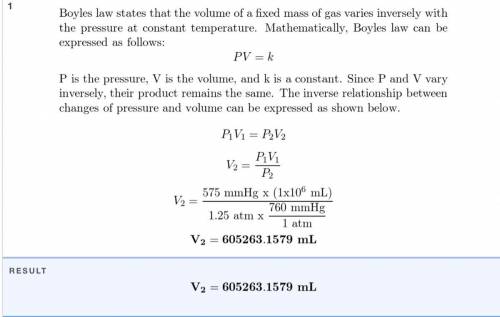
Chemistry, 30.03.2021 09:00, hgdthbgjnb83661
A sample of oxygen that occupies 2.9 X
10-6 mL at 635 mm Hg is subjected to a
pressure of 1.26 atm. What will the final vol-
ume of the sample be if the temperature is
held constant?
Answer in units of mL.
help please please


Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 11:00, RidhaH
Which statement correctly identifies the scientific question and describes why the question is scientific? question 1 refers to the supernatural. question 2 reflects a moral or social value. question 3 refers to something that can be measured. question 4 reflects a question that can’t be observed.
Answers: 1

Do you know the correct answer?
A sample of oxygen that occupies 2.9 X
10-6 mL at 635 mm Hg is subjected to a
pressure of 1.2...
pressure of 1.2...
Questions in other subjects:



Mathematics, 17.11.2020 19:50

Health, 17.11.2020 19:50

Mathematics, 17.11.2020 19:50