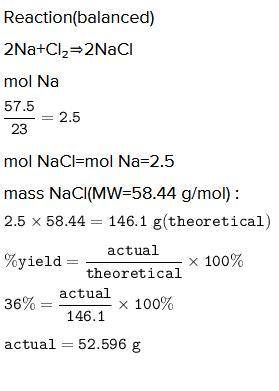Chemistry, 29.03.2021 19:20, joyceslater16
A chemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. The chemical reaction that occurred is shown.
Na + Cl2 → NaCl
If the percentage yield of the reaction is 86%, what is the actual yield? Show your work, including the use of stoichiometric calculations and conversion factors.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 05:50, mrylenastewart
What are transitions between a liquid and a solid called? identify which way they are transitioning
Answers: 2

Chemistry, 22.06.2019 09:20, nyceastcoast
Give the orbital configuration of the phosphorus (p) atom.
Answers: 1

Chemistry, 22.06.2019 09:20, payshencec21
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 22.06.2019 10:10, babyphoraaaaa
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate, m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
Do you know the correct answer?
A chemist reacted 57.50 grams of sodium metal with an excess amount of chlorine gas. The chemical re...
Questions in other subjects:

English, 11.11.2019 12:31

Mathematics, 11.11.2019 12:31





Mathematics, 11.11.2019 12:31

Mathematics, 11.11.2019 12:31

Biology, 11.11.2019 12:31