
Chemistry, 28.03.2021 19:20, gwendallinesikes
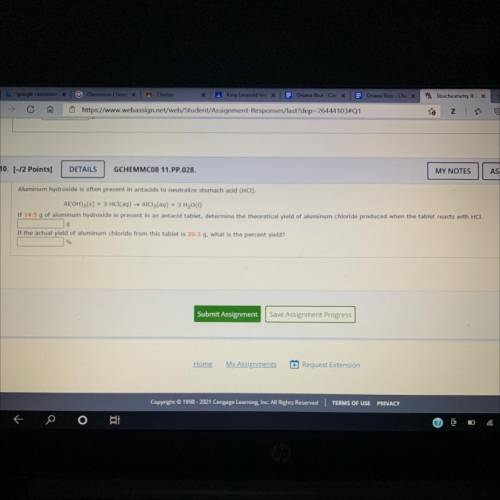
Aluminum hydroxide is often presant in antacids to neutralize stomach (HCI) Al(OH) 3 (s)+3HCl(aq) AlCl 3 (aq)+3H 2 O(l) is present in an antacid tablet, the theoretical yield of aluminum chloride produced the tablet reacts with HCH the actual yield of aluminum chloride from this tablet what the percent yield? SOMEONE PLEASE HELP!!

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, junkmailemail42
Which element is the least metallic between cadmium, silver, zinc, or iron?
Answers: 1

Chemistry, 23.06.2019 04:10, angelina6836
Which of the following is described by the equation h2o(s)+ heat=h2o(i) a freezing melting condensing evaporating
Answers: 2

Chemistry, 23.06.2019 06:20, cowboo5000pcl655
An object of mass 10.0 kg and volume 1000 ml and density 10 g/ml sinks in water who’s density is 1.0 g/ml. what is the mass of the water which has been displaced in kilograms
Answers: 1

Chemistry, 23.06.2019 07:00, asims13
The following transition occurs at a molecular level for a substance. what transition corresponds to this change in microscopic structure? the carbon dioxide molecules on the left are in a regular, tightly packed pattern. after heating, it becomes much lower density. a. melting b. boiling c. sublimation d. freezing
Answers: 1
Do you know the correct answer?
Aluminum hydroxide is often presant in antacids to neutralize stomach (HCI) Al(OH) 3 (s)+3HCl(aq) Al...
Questions in other subjects:


Mathematics, 27.08.2019 01:50

Mathematics, 27.08.2019 01:50


Mathematics, 27.08.2019 01:50


History, 27.08.2019 01:50


Mathematics, 27.08.2019 01:50







