
Chemistry, 28.03.2021 14:00, titalili0204
A water solution containing an unknown quantity of a
nonelectrolyte solute is found to have a freezing point of
–0.23°C. What is the molal concentration of the solution?

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 23.06.2019 02:00, Hellopeople233
Which of the following substances is the most soluble in water? a. sodium chloride b. methane c. bromine d. carbon
Answers: 1

Chemistry, 23.06.2019 05:00, cpcoolestkid4
C=59(f−32)the equation above shows how temperature f, measured in degrees fahrenheit, relates to a temperature c, measured in degrees celsius. based on the equation, which of the following must be true? a temperature increase of 1 degree fahrenheit is equivalent to a temperature increase of 59 degree celsius. a temperature increase of 1 degree celsius is equivalent to a temperature increase of 1.8 degrees fahrenheit. a temperature increase of 59 degree fahrenheit is equivalent to a temperature increase of 1 degree celsius. a) i onlyb) ii onlyc) iii onlyd) i and ii only
Answers: 1
Do you know the correct answer?
A water solution containing an unknown quantity of a
nonelectrolyte solute is found to have a freez...
Questions in other subjects:


English, 30.09.2019 15:30

Mathematics, 30.09.2019 15:30







Chemistry, 30.09.2019 15:30


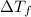 =
=  · b·i
· b·i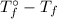
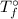 = The freezing point of pure water = 0°C
= The freezing point of pure water = 0°C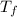 = The freezing point of the solution = -0.23°C
= The freezing point of the solution = -0.23°C



