

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 22:00, creepycrepes
Which function is performed by earths atmosphere? a. ultraviolet rays are prevented from reaching the ozone layer. b. earths temperature is raised and moderated by trapping in heat c. charged particles from the sun are prevented from reaching earth. d. magnetic charges from space are prevented from reaching earths surface.
Answers: 2

Chemistry, 22.06.2019 06:00, tddreviews
Match the name of the following compound: mgso4 · h2omagnesium sulfate monohydratemagnesium (ii) sulfate monohydratemagnesium (ii) sulfate hydratemagnesium sulfate hydrate
Answers: 1

Chemistry, 22.06.2019 10:50, mi364
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3
Do you know the correct answer?
Acid spills are often neutralized with sodium carbonate. For example
Na CO2 (s) + H2SO4 (aq) → Na2S...
Questions in other subjects:

Engineering, 08.05.2021 01:00



History, 08.05.2021 01:00


Social Studies, 08.05.2021 01:00


Mathematics, 08.05.2021 01:00


Mathematics, 08.05.2021 01:00


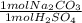 = 45 mol NaCO₂
= 45 mol NaCO₂



