
Rank the following carboxylic acids by acid strength, with the strongest at the top and the weakest at the bottom. It may help to draw each Lewis structure. Drag and drop options into correct order. For keyboard navigation...SHOW MORE Press space or enter to grab A. CF3CO2H B. CHF2CO2HC. CH2FCO2H D. CH3CO2H

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 20:00, Chynadoll94
Iam hoping to create 5.72 grams of glucose. the plant was given 4.75 liters of co2 and 2.81 g of h20. which reactant was the limiting reagent? how much excess mass did we have of the other reactant?
Answers: 1

Chemistry, 22.06.2019 21:00, rah45
Which of these is an example of pseudoscience? a) predicting the time of sunrise based on data on position of earth b) predicting the date of the moon phases based on data on position of earth c) predicting eclipses based on the position of the sun and the moon d) predicting future events in a person's life based on the position of the moon
Answers: 1

Chemistry, 23.06.2019 07:00, Bassoonist
How does science use models to gain a better understanding of concepts?
Answers: 1

Chemistry, 23.06.2019 12:30, floihqrrigweo
How do you interpret a chromatogram for what mixtures contain?
Answers: 3
Do you know the correct answer?
Rank the following carboxylic acids by acid strength, with the strongest at the top and the weakest...
Questions in other subjects:




Chemistry, 01.07.2020 15:01


Physics, 01.07.2020 15:01


Mathematics, 01.07.2020 15:01



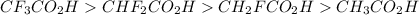
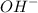 groups in the COOH group towards itself, making it relatively easy to loose the proton of the carboxyl group.
groups in the COOH group towards itself, making it relatively easy to loose the proton of the carboxyl group.



