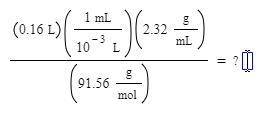(0.16L) (1mL/10^-3L) (2.32g/mL)/ (91.56 g/mol)
...

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:10, vapelordcarl69
When 225mg of anthracene, c14h10(s), was burned in a bomb calorimeter the temperature rose by 1.75k. calculate the calorimeter constant. by how much will the temperature rise when 125mg of phenol, c6h5oh(s), is burned in the calorimeter under the same conditions? (δch< (c14h10,s)=–7061 kj mol−1.)
Answers: 3

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 16:00, bbrogle5154
If 15 drops of ethanol from a medical dropper weight 0.60g, how many drops does it takes from a dropper to dispense 1.0ml of ethanol? the density of ethanol is 0.80g/ml
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 01.12.2020 22:20

Mathematics, 01.12.2020 22:20

History, 01.12.2020 22:20

Mathematics, 01.12.2020 22:20

Physics, 01.12.2020 22:20

History, 01.12.2020 22:20

Mathematics, 01.12.2020 22:20


Mathematics, 01.12.2020 22:20