
Chemistry, 26.03.2021 23:00, elainelytal1508
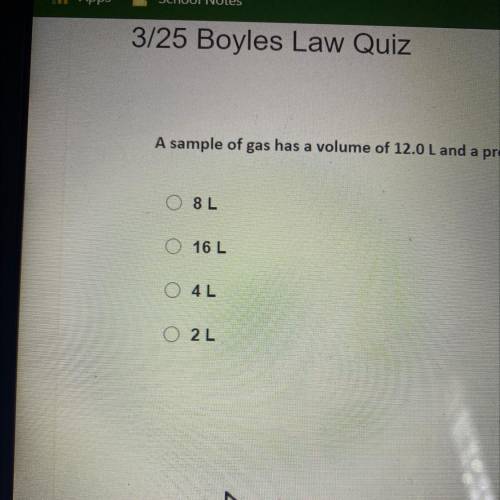
A sample of gas has a volume of 12.0 L and a pressure of 2.00 ATM. If the pressure of gas is increased to6.00 ATM, what is the new volume of the gas

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 20:30, lemonsalt9378
14. complete and balance the equations for the single displacement reactions. a. zn + pb(no3)2 -> b. al + niso4 -> 15. complete and balance the equations for the double displacement reactions. a. agno3(aq) + nacl(aq) -> b. mg(no3)2(aq) + koh(aq) -> 16. complete and balance the equations for the combustion reactions. a. __ ch4 + o2 -> b. __ c3h6 + o2 -> c. + o2 ->
Answers: 2


Chemistry, 22.06.2019 12:40, valenzueladomipay09u
How does concentration affect reaction rate
Answers: 2
Do you know the correct answer?
A sample of gas has a volume of 12.0 L and a pressure of 2.00 ATM. If the pressure of gas is increas...
Questions in other subjects:

Mathematics, 24.04.2020 04:25

Biology, 24.04.2020 04:25




Mathematics, 24.04.2020 04:25

Mathematics, 24.04.2020 04:25









