
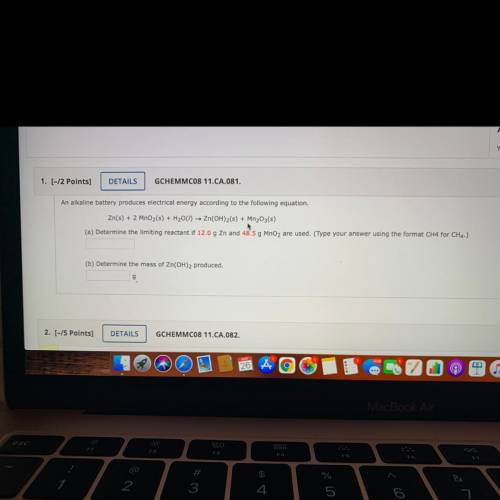
An alkaline battery produces electrical energy according to the following equation.
Zn(s) + 2 MnO2(s) + H2001) — Zn(OH)2(s) + Mn2O3(s)
(a) Determine the limiting reactant if 12.0 g Zn and 48.5 g MnO2 are used. (Type your answer using the format CH4 for CH4.)
(b) Determine the mass of Zn(OH)2 produced.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 17:20, phanuel642
The small bags of silica gel you often see in a new shoe box are placed there to control humidity. despite its name, silica gel is a solid. it is a chemically inert, highly porous, amorphous form of sio2. because water vapor readily adsorbs onto the surface of silica gel, it acts as a desiccant. despite not knowing mechanistic details of the adsorption of water onto silica gel, from the information provided you should be able to make an educated guess about the thermodynamic characteristics of the process. predict the signs for δg, δh, and δs for the adsorption of water.
Answers: 2


Chemistry, 23.06.2019 06:30, tdahna0403
The molar mass of cu is 63.55 g/mol. the number of grams of cu produced in this reaction is
Answers: 3

Chemistry, 23.06.2019 16:00, crystaldewar55C
What is the consequence on your brain for snorting or taking amphetamines(meth/stimulant) as a 16 year old(minor)?
Answers: 2
Do you know the correct answer?
An alkaline battery produces electrical energy according to the following equation.
Zn(s) + 2 MnO2(...
Questions in other subjects:

History, 22.05.2020 08:59


Mathematics, 22.05.2020 08:59

English, 22.05.2020 08:59



Biology, 22.05.2020 08:59









