Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, natannale
Darla claims that the first periodic table developed by mendeleev was not completely accurate, so it is not useful at all. harmony argues that it establish the periodic table we use today, making it more credible. who is correct and why? darla is correct, because a model that has any mistakes should be thrown out. darla is correct, because a good model would not need to change. harmony is correct, because mendeleev’s model had all of the information correct in the first version. harmony is correct, because mendeleev’s model made predictions that came true.
Answers: 1


Chemistry, 22.06.2019 14:30, hjlhdjfhjh
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1

Chemistry, 22.06.2019 14:30, malenacastillo4887
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
Do you know the correct answer?
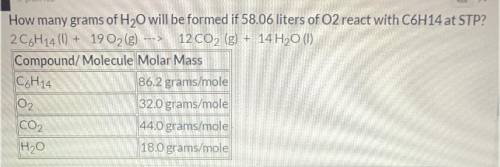
How many grams of H2O will be formed if 58.06 liters of O2 react with C6H14 at STP?
Balanced equati...
Questions in other subjects:

Spanish, 29.06.2019 21:30

Mathematics, 29.06.2019 21:30

Mathematics, 29.06.2019 21:30





Mathematics, 29.06.2019 21:30

Mathematics, 29.06.2019 21:30