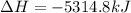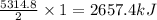Chemistry, 26.03.2021 01:00, turboslayer
Butane C4H10 (g),(Hf = –125.7), combusts in the presence of oxygen to form CO2 (g) (Delta. Hf = –393.5 kJ/mol), and H2O(g) (Delta. Hf = –241.82) in the reaction: 2 upper C subscript 4 upper H subscript 10 (g) plus 13 upper O subscript 2 (g) right arrow 8 upper C upper O subscript 2 plus 10 upper H subscript 2 upper O (g). What is the enthalpy of combustion, per mole, of butane? Use Delta H r x n equals the sum of delta H f of all the products minus the sum of delta H f of all the reactants.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:40, janelisse199820
Non renewable resources like petroleum eventually
Answers: 2

Chemistry, 22.06.2019 14:10, cameronbeaugh
13. a covalent bond between two atoms is likely to be polar if: a. one of the atoms is much more electronegative than the other. b. the two atoms are equally electronegative. c. the two atoms are of the same element. d. the bond is part of a tetrahedrally shaped molecule. e. one atom is an anion.
Answers: 1

Chemistry, 22.06.2019 23:00, jolainjoseph01998
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 01:00, carson9373
Wind and moving water provide energy. chemical mechanical thermal none of the above
Answers: 1
Do you know the correct answer?
Butane C4H10 (g),(Hf = –125.7), combusts in the presence of oxygen to form CO2 (g) (Delta. Hf = –393...
Questions in other subjects:




Mathematics, 19.10.2019 18:30

Social Studies, 19.10.2019 18:30


Mathematics, 19.10.2019 18:30



French, 19.10.2019 18:30


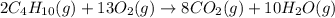
![\Delta H=[n\times H_f_{products}]-[n\times H_f_{reactants}]](/tpl/images/1221/8923/4f68b.png)
![\Delta H=[8\times H_f_{CO_2}+10\times H_f_{H_2O}]-[2\times H_f_{C_4H_{10}+13\times H_f_{O_2}}]](/tpl/images/1221/8923/e94db.png)
![\Delta H=[(8\times -393.5)+(10\times -241.82)]-[(2\times -125.7)+(13\times 0)]](/tpl/images/1221/8923/8343f.png)