
Chemistry, 26.03.2021 01:00, kaliyab191
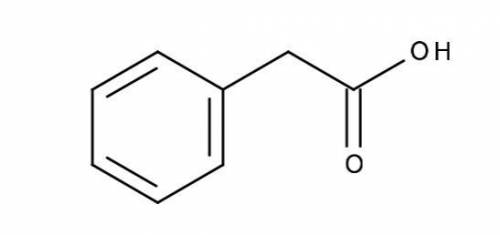
Calculate (a) the pH of a 0.0250 M solution of phenylacetic acid, and (b) the pH of a 0.0500 M solution of sodium phenylacetate. The pKa of phenylacetic acid is 4.31, and its structure is shown below.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, annsmith66
What statement goes against the kinetic theory of gases
Answers: 1

Chemistry, 22.06.2019 20:00, bbyjean9974
State one important difference between a physical change and a chemical change?
Answers: 1
Do you know the correct answer?
Calculate (a) the pH of a 0.0250 M solution of phenylacetic acid, and (b) the pH of a 0.0500 M solut...
Questions in other subjects:

English, 20.05.2020 10:58

History, 20.05.2020 10:58


English, 20.05.2020 10:58

Mathematics, 20.05.2020 10:58

English, 20.05.2020 10:58




Mathematics, 20.05.2020 10:58






