The information below describes a redox reaction.
Ag+ (aq) + Al(s) -> Ag(s) + Al^3+ (aq)
<...

Chemistry, 25.03.2021 22:10, iesps010411
The information below describes a redox reaction.
Ag+ (aq) + Al(s) -> Ag(s) + Al^3+ (aq)
Ag+ (aq) + e^- -> Ag(s)
Al(s) -> Al^3+ (aq) + 3e^-
What is the coefficient of silver in the final, balanced equation for this reaction?
A. 1
B. 2
C. 3
D. 4
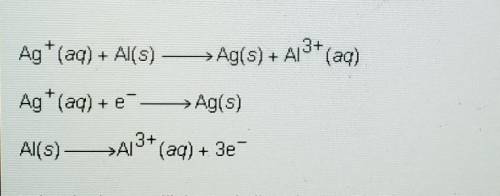

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 21:40, taysomoneyyy
During trial 2, what allowed you to determine that aluminum was the limiting reactant? check all that apply. all of the copper dissolved. all of the aluminum dissolved. the solution turned clear. the number of grams of copper(ii) chloride used in the reaction was greater than the number of grams of aluminum. the molar ratio of copper(ii) chloride to aluminum was greater than 3: 2, the equation’s molar ratio.
Answers: 2


Chemistry, 22.06.2019 09:50, bridgetosanders
What are four significant sources of ghgs that come from wostem washington?
Answers: 2

Do you know the correct answer?
Questions in other subjects:

Mathematics, 05.12.2021 04:50

Physics, 05.12.2021 04:50

Mathematics, 05.12.2021 04:50


Mathematics, 05.12.2021 05:00


English, 05.12.2021 05:00

Physics, 05.12.2021 05:00

Mathematics, 05.12.2021 05:00

Mathematics, 05.12.2021 05:00






