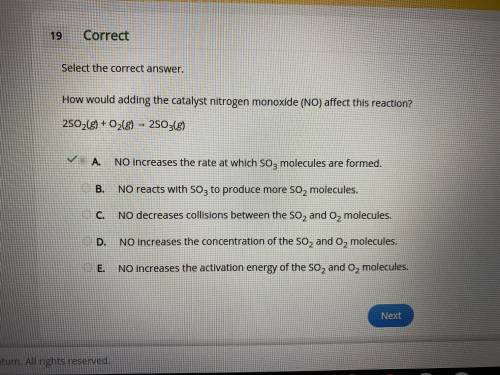
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g)
A.
NO increases the rate at which SO3 molecules are formed.
B.
NO reacts with SO3 to produce more SO2 molecules.
C.
NO decreases collisions between the SO2 and O2 molecules.
D.
NO increases the concentration of the SO2 and O2 molecules.
E.
NO increases the activation energy of the SO2 and O2 molecules

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 21:20, skyemichellec
40dm3 of gas at 760 torr are heated from 5°c to 50°c what is the new volume
Answers: 3

Chemistry, 22.06.2019 22:30, medinajocelyn45
Which compound most likely has the greatest bond energy?
Answers: 2
Do you know the correct answer?
How would adding the catalyst nitrogen monoxide (NO) affect this reaction?
2SO2(g) + O2(g) → 2SO3(g...
Questions in other subjects:


Social Studies, 08.02.2021 21:30


Mathematics, 08.02.2021 21:30


Mathematics, 08.02.2021 21:30


French, 08.02.2021 21:30

Mathematics, 08.02.2021 21:30

Mathematics, 08.02.2021 21:30