
Chemistry, 24.03.2021 20:40, dounutalien
Which of the following gas samples would contain the same amount of gas as 200 mL of helium, He(g), at 25° C and 1 atm?
Select one:
a. all the above
b. 200 mL of neon, Ne (g), at 25° C and 1 atm
c. 40 mL of methane, CH4 (g), at 25° C and 1 atm
d. 400 mL of hydrogen, H2 (g), at 25° C and 1 atm
Need done asap! Will give 5 stars!

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 04:00, lucasrandall
Asolution contains 225 g of sodium chloride, nacl, dissolved in enough water to make a 0.25 l of solution. what is the molarity of the solution?
Answers: 2


Do you know the correct answer?
Which of the following gas samples would contain the same amount of gas as 200 mL of helium, He(g),...
Questions in other subjects:


Computers and Technology, 25.07.2019 23:00

History, 25.07.2019 23:00


Health, 25.07.2019 23:00


Physics, 25.07.2019 23:00




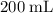 of neon
of neon 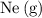 at
at 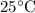 and
and 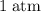 (the second choice) would contain an equal number of gas particles as
(the second choice) would contain an equal number of gas particles as 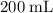 of
of 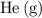 at
at 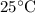 and
and 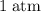 (assuming that all four gas samples behave like ideal gases.)
(assuming that all four gas samples behave like ideal gases.) 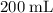 . Hence, that choice would be the only one with as many gas particles as
. Hence, that choice would be the only one with as many gas particles as 



