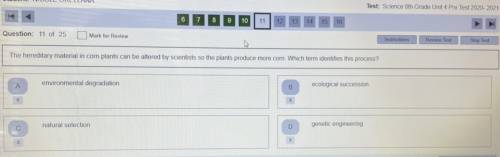
Please help! This is for a test and I want to have a good grade. Have a great day! :)
...


Answers: 1
Other questions on the subject: Chemistry


Chemistry, 23.06.2019 06:00, hopechinn6646
Complete the sentences to best explain the ranking. match the words below to the appropriate blanks in the sentences. a less polar bondhigher molar massion-dipole forcesstronger intermolecular forcesdipole-dipole forcesdispersion forceshydrogen bonding1. h2s and h2se exhibit the following intermolecular forces:.2. therefore, when comparing h2s and h2se the one with a has a higher boiling point .3. the strongest intermolecular force exhibited by h2o is . therefore, when comparing h2se and h2o the one with has a higher boiling point.
Answers: 1

Chemistry, 23.06.2019 09:20, jahnasiahill5349
Description: biological systems 5 differ from chemical systems when it comes to equilibrium. in a chemical system once equilibrium is met, no other reactions occur. in a biological system, a dynamic equilibrium is used when a substrate is turned into a product, another reaction creates the same substrate thus keeping the concentrations stagnant. this allows for cells to continually make new compounds without messing the delta g for the systems instructions: write a response to the following prompt and then respond to your peers. your individual response is due thursday at midnight (cst) your response to your peers is due saturday at midnight (cst) prompt: propose what would happen if a living cell all the sudden reached chemical equilibrium. also discuss the effects of build up of a particular substrate on a biological system. how would this affect overall delta g's?
Answers: 3

Chemistry, 23.06.2019 10:00, Sariyahhall1
Two moles of potassium chloride and three moles of oxygen are produced from the decomposition of two moles of potassium chlorate, kcos3(s). write the balanced equation. how many moles of oxygen are produced from 12 moles of potassium chlorate
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Health, 02.08.2019 14:30

Biology, 02.08.2019 14:30


Social Studies, 02.08.2019 14:30


History, 02.08.2019 14:30

History, 02.08.2019 14:30

Mathematics, 02.08.2019 14:30

English, 02.08.2019 14:30