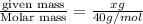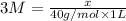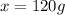How would you prepare a 1 L solution of 3 M Mgo?
O A. Put 120 grams of Mgo in the beaker and add exactly 1 L of water.
O B. Put 3 grams of Mgo in the beaker and add exactly 1 L of water.
O C. Put 3 grams of Mgo in the beaker and add enough water to reach the 1 L mark.
O D. Put 120 grams of Mgo in the beaker and add enough water to reach the 1 L mark.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 14:10, gizmo50245
Calculate the mass percent of hydrogen in methyl acetate
Answers: 1


Chemistry, 22.06.2019 14:30, joejoefofana
Consider the reduction reactions and their equilibrium constants. cu+(aq)+e−↽−−⇀cu(s)pb2+(aq)+2e−↽−−⇀ pb(s)fe3+(aq)+3e−↽−−⇀fe(=6.2×108=4. 0×10−5=9.3×10−3 cu + ( aq ) + e − ↽ − − ⇀ cu ( s ) k =6.2× 10 8 pb 2 + ( aq ) +2 e − ↽ − − ⇀ pb ( s ) k =4.0× 10 − 5 fe 3 + ( aq ) +3 e − ↽ − − ⇀ fe ( s ) k =9.3× 10 − 3 arrange these ions from strongest to weakest oxidizing agent.
Answers: 3

Chemistry, 22.06.2019 20:00, jalenevoyles
Phosphoric acid is a triprotic acid ( =6.9×10−3 , =6.2×10−8 , and =4.8×10−13 ). to find the ph of a buffer composed of h2po−4(aq) and hpo2−4(aq) , which p value should be used in the henderson–hasselbalch equation? p k a1 = 2.16 p k a2 = 7.21 p k a3 = 12.32 calculate the ph of a buffer solution obtained by dissolving 18.0 18.0 g of kh2po4(s) kh 2 po 4 ( s ) and 33.0 33.0 g of na2hpo4(s) na 2 hpo 4 ( s ) in water and then diluting to 1.00 l.
Answers: 3
Do you know the correct answer?
How would you prepare a 1 L solution of 3 M Mgo?
O A. Put 120 grams of Mgo in the beaker and add ex...
Questions in other subjects:

Chemistry, 31.05.2021 14:00

Chemistry, 31.05.2021 14:00


Mathematics, 31.05.2021 14:00

English, 31.05.2021 14:00

Arts, 31.05.2021 14:00

Business, 31.05.2021 14:00



Mathematics, 31.05.2021 14:00


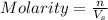
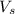 = volume of solution in L
= volume of solution in L
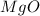 =
=