
Chemistry, 23.03.2021 02:40, willowcollins3753
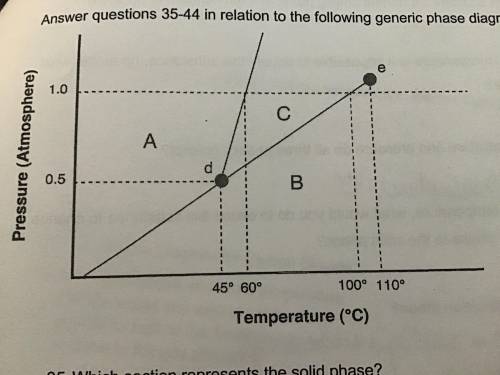
1. Above what temperature is it impossible to liquify this substance, no matter what the pressure? 2. At a constant temperature, what would you do to cause this substance to change from the liquid phase to the solid phase?

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 10:30, cheyennecarrillo14
If you add 5.00 ml of 0.100 m sodium hydroxide to 50.0 ml of acetate buffer that is 0.100 m in both acetic acid and sodium acetate, what is the ph of the resulting solution? acetic acid: ka = 1.8. x 10-5
Answers: 1

Chemistry, 22.06.2019 14:00, ashlynneboogs0056
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 15:00, alanmarcus22
What does the symbol (–hfus) indicate in a phase change?
Answers: 1
Do you know the correct answer?
1. Above what temperature is it impossible to liquify this substance, no matter what the pressure?...
Questions in other subjects:




History, 24.02.2021 16:50





World Languages, 24.02.2021 16:50

Advanced Placement (AP), 24.02.2021 16:50






