
Ammonium perchlorate NH4ClO4 is a powerful solid rocket fuel, used in the Space Shuttle boosters. It decomposes into nitrogen N2 gas, chlorine Cl2 gas, oxygen O2 gas and water vapor, releasing a great deal of energy. Calculate the moles of oxygen produced by the reaction of 0.055mol of ammonium perchlorate. Be sure your answer has a unit symbol, if necessary, and round it to the correct number of significant digits.

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, josmanu235
Why is permeable soil best for plants that need a lot of drainage?
Answers: 1

Chemistry, 22.06.2019 09:20, lanaiheart7
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
Do you know the correct answer?
Ammonium perchlorate NH4ClO4 is a powerful solid rocket fuel, used in the Space Shuttle boosters. It...
Questions in other subjects:

Biology, 23.04.2021 20:10

History, 23.04.2021 20:10

Mathematics, 23.04.2021 20:10


Mathematics, 23.04.2021 20:10



Arts, 23.04.2021 20:10



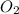 are produced by the reaction of 0.055 mol of ammonium perchlorate.
are produced by the reaction of 0.055 mol of ammonium perchlorate.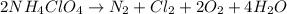
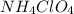 produce = 2 moles of
produce = 2 moles of 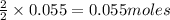 of
of 



