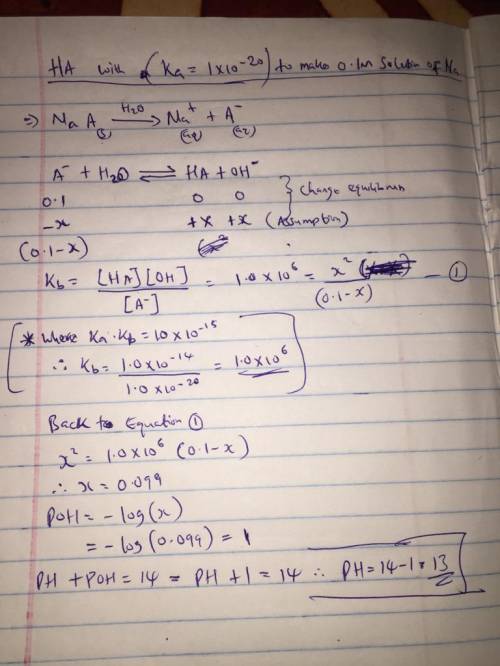Chemistry, 22.03.2021 05:40, HOTaco2181
Consider an exceptionally weak acid, HA, with a Ka = 1x10 -20 . You make a 0.1M solution of the salt Na

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 15:30, tiniecisneros28
Complete this brønsted-lowry reaction placing each product by its appropriate label. hso4- + hcn
Answers: 1

Chemistry, 22.06.2019 05:30, alaynagrace1111
What is the mass of each element in a 324.8 sample of co2
Answers: 1

Chemistry, 22.06.2019 09:30, matpakootas521
Why do cells appear different in distilled water than they do in 10% salt water?
Answers: 2

Chemistry, 22.06.2019 20:00, kalcius9698
What is the molarity of the solution produced when 145 g of nacl is dissolved in sufficient water to prepare 2.75 l of solution?
Answers: 1
Do you know the correct answer?
Consider an exceptionally weak acid, HA, with a Ka = 1x10 -20 . You make a 0.1M solution of the salt...
Questions in other subjects:

Chemistry, 06.02.2021 05:10

English, 06.02.2021 05:10

Physics, 06.02.2021 05:10

Mathematics, 06.02.2021 05:10




Mathematics, 06.02.2021 05:10

Mathematics, 06.02.2021 05:10