
Chemistry, 22.03.2021 04:20, jayjinks976
If it takes 38.70 cm^3 of 1.90M NaOH to neutralize 10.30cm^3 of H2SO4 in a battery. What is the concentration of the H2SO4?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 22:10, zwbaby3693
Which aqueous solution of ki freezes at the lowest temperature? 1) 1 mol of ki in 500. g of water 2) 2 mol of ki in 500. g of water 3) 1 mol of ki in 1000. g of water 4) 2 mol of ki in 1000. g of water
Answers: 3

Chemistry, 23.06.2019 02:30, paulinahunl17
What type of energy conversion occurs when you place your feet near the fire place and they become warm
Answers: 1

Chemistry, 23.06.2019 02:40, towelmearowel
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
Do you know the correct answer?
If it takes 38.70 cm^3 of 1.90M NaOH to neutralize 10.30cm^3 of H2SO4 in a battery. What is the conc...
Questions in other subjects:


Physics, 26.03.2021 07:00

Mathematics, 26.03.2021 07:00

Mathematics, 26.03.2021 07:10

Mathematics, 26.03.2021 07:10

Physics, 26.03.2021 07:10

History, 26.03.2021 07:10


Biology, 26.03.2021 07:10


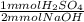 = 36.765 mmol H₂SO₄
= 36.765 mmol H₂SO₄



