
Chemistry, 21.03.2021 21:20, HOPING4632
The first order decomposition of N2O5 to form NO2 and O2, has k =
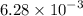
s^-1 at 70°C.
What % of the NO2 has decomposed after 278 s?

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:10, cristinaledford3696
Imagine that you have produced several versions of lactase, each of which differs from normal lactase by a single amino acid. describe a test that could indirectly determine which of the versions significantly alters the three-dimensional shape of the lactase protein.
Answers: 2


Chemistry, 22.06.2019 14:50, ladybugperez05
Which of the following is most likely true about water in chemical systems? a) water dissolves nonpolar ionic compounds. b) water dissociates ionic compounds. c) water dissociates covalent molecules. d) water dissolves nonpolar covalent substances.
Answers: 1

Chemistry, 22.06.2019 21:00, melissalopez12
Acandle’s wick is the fabric string that holds the flame, and it burns down at a constant slow pace when the candle is lit. the wick is usually surrounded by wax. which is the most important property of covalent compounds that makes them useful for making candle wax? a low boiling point a low melting point a high boiling point a high melting point
Answers: 1
Do you know the correct answer?
The first order decomposition of N2O5 to form NO2 and O2, has k =
s^-1 at 70°C.
What % o...
s^-1 at 70°C.
What % o...
Questions in other subjects:




Mathematics, 25.02.2021 22:10

History, 25.02.2021 22:10



History, 25.02.2021 22:10

Mathematics, 25.02.2021 22:10

Social Studies, 25.02.2021 22:10






