
Chemistry, 19.03.2021 21:40, lilquongohard
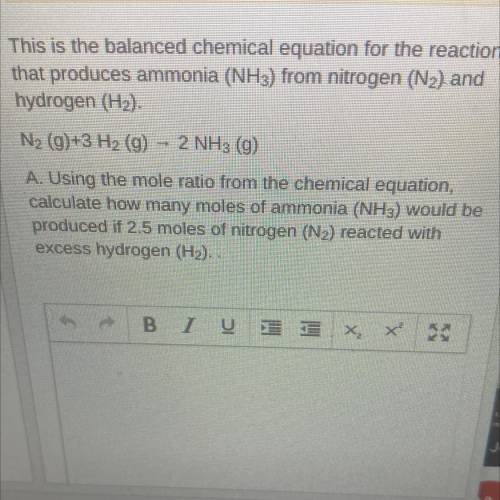
This is the balanced chemical equation for the reaction that produces ammonia (NH3) from nitrogen (N2) and hydrogen (H2).
N2 (g)+3 H2 (g) - 2 NH3 (g)
A. Using the mole ratio from the chemical equation, calculate how many moles of ammonia (NH3) would be produced if 2.5 moles of nitrogen (N2) reacted with
excess hydrogen (H2). .

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 13:30, estherdinhllama
Which of these best describes the scientific process
Answers: 3

Chemistry, 21.06.2019 22:30, larreanathalie3523
The diagram shows the structures of horse and cat forelimbs. what does the diagram suggest about the evolutionary relationship between these two mammals? a. they have homologous structures, indicating a common ancestor. b. they have analogous structures, indicating a common ancestor. c. they have homologous structures, indicating that they do not have a common ancestor. d. they have analogous structures, indicating that they do not have a common ancestor.
Answers: 2

Chemistry, 22.06.2019 15:00, YoVeoAnime
20 pts ‼️ an unmanned spacecraft travels to mars. mars has a lower strength of gravity than earth. where in the image is the spacecraft’s weight the greatest?
Answers: 1
Do you know the correct answer?
This is the balanced chemical equation for the reaction that produces ammonia (NH3) from nitrogen (N...
Questions in other subjects:

SAT, 29.01.2021 23:10

Biology, 29.01.2021 23:10




Social Studies, 29.01.2021 23:10



Mathematics, 29.01.2021 23:10







