
Potassium chlorate, a common oxidizing agent in fireworks and matchheads, undergoes a solid-state disproportionation reaction when heated:
4KClO3 (s) ⟶ Δ3KClO4 (s) + KCl (s).
Use ΔHf ° and S° values to calculate ΔG_sys ° (which is ΔGrxn °) in kJ at 25°C for this reaction.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:30, ashleybarrera2000
For each of the following compounds, decide whether the compound's solubility in aqueous solution changes with ph. if the solubility does change, pick the ph at which you'd expect the highest solubility. you'll find ksp data in the aleks data tab. compounds does solubility change with ph
Answers: 3


Chemistry, 23.06.2019 09:00, ashhull2002
Need ! assume that the variables x and y are directly related. if k = 8, what is the value for each of the following points? be sure and record your data to be used in the following problem. x y k 0.
Answers: 2
Do you know the correct answer?
Potassium chlorate, a common oxidizing agent in fireworks and matchheads, undergoes a solid-state di...
Questions in other subjects:


Spanish, 02.12.2021 23:20

Business, 02.12.2021 23:20


History, 02.12.2021 23:20



Physics, 02.12.2021 23:20

Computers and Technology, 02.12.2021 23:20

Mathematics, 02.12.2021 23:20


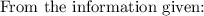
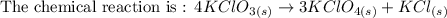

![\implies G^0_{rxn} = 3 \times \Delta _fG^0 [KClO_4{(s)}] + \Delta_fG^0[KCl_{(s)}] - 4 \times \Delta _f G^0 [ KClO_3 (s) ]](/tpl/images/1206/8925/2b60b.png)
![\Delta _fG^0 \ values \ at \ 25^0 \ C (298 \ K) are\ given \ as:\\\\ \Delta _fG^0 [KClO_4(s)] = -303.09 \ kJ \\ \\ \Delta _fG^0 [KCl(s) ] = - 409.14 \ kJ \\ \\ \Delta_f G^0 [KClO_3_{(s)}] = -296.25 \ kJ \\ \\ replacing \ the \ above \ values \ into \ equation (1) ; then:\\ \\ \\\Delta G^0_{rxn} = 3 *(-303.09) + (-409.14) - 4*(-296.25) \ kJ \\ \\ = (-909.27 - 409.14 + 1185) \ kJ \\ \\ = -133.41 \ kJ \\ \\ \mathbf{\Delta G^0_{rxn} = -133.4 \ kJ }](/tpl/images/1206/8925/f4981.png)





