
Consider the reactivity of phenol, bromobenzene, toluene, and nitrobenzene toward electrophilic aromatic substitution.
The most reactive compound is:
because the character of the increases the rate of the reaction.
The least reactive compound is
because the character of the decreases the rate of the reaction.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, angelinararr5783
Which answer lists the fundamental forces in order from strongest to weakest
Answers: 1

Chemistry, 22.06.2019 01:40, georgehall3027
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 13:00, wbrandi118
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 17:10, hahahwha
Acalorimeter is to be calibrated: 51.203 g of water at 55.2 degree c is added to a calorimeter containing 49.783 g of water at 23.5c. after stirring and waiting for the system to equilibrate, the final temperature reached is 37.6 degree c. specific heat capacity of water (s = 4.18 j/g∙degree c). calculate the calorimeter constant. (smδt)warm water = -[(smδt)cold water + (calorimeterδtcold water)]
Answers: 2
Do you know the correct answer?
Consider the reactivity of phenol, bromobenzene, toluene, and nitrobenzene toward electrophilic arom...
Questions in other subjects:


Mathematics, 22.04.2021 20:50

Mathematics, 22.04.2021 20:50


English, 22.04.2021 20:50

Mathematics, 22.04.2021 20:50

English, 22.04.2021 20:50




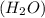 in which one of the hydrogen atoms has been substituted by an alkyl group, which in organic structures is usually expressed by R.NITROBENZENE-:The organic compound nitrobenzene has the chemical formula
in which one of the hydrogen atoms has been substituted by an alkyl group, which in organic structures is usually expressed by R.NITROBENZENE-:The organic compound nitrobenzene has the chemical formula 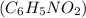 . It's a pale yellow oil that's insoluble in water and smells like almonds. Greenish-yellow crystals form when it freezes. It is made on a wide scale as a precursor to aniline from benzene. It is sometimes used as a solvent in the laboratory, especially for electrophilic reagents.ELECTRON WITHDRAWING GROUP-: An electron withdrawing group (EWG) is a type of group that reduces electron density in a molecule by bonding to a carbon atom. EWGs alter a molecule's reactivity by reducing electron density on neighboring carbon atoms.NITRO GROUP-: The nitro group is one of the most widely used explosophores (functional groups that combine to form a compound explosive). In addition, the nitro group is a heavy electron-withdrawing group. CH bonds alpha (adjacent) to the nitro group may be acidic due to this property.
. It's a pale yellow oil that's insoluble in water and smells like almonds. Greenish-yellow crystals form when it freezes. It is made on a wide scale as a precursor to aniline from benzene. It is sometimes used as a solvent in the laboratory, especially for electrophilic reagents.ELECTRON WITHDRAWING GROUP-: An electron withdrawing group (EWG) is a type of group that reduces electron density in a molecule by bonding to a carbon atom. EWGs alter a molecule's reactivity by reducing electron density on neighboring carbon atoms.NITRO GROUP-: The nitro group is one of the most widely used explosophores (functional groups that combine to form a compound explosive). In addition, the nitro group is a heavy electron-withdrawing group. CH bonds alpha (adjacent) to the nitro group may be acidic due to this property.




