
Chemistry, 19.03.2021 02:40, cheefdogg420
Liquids B and C are partially miscible at 25 oC. When one starts with 1 mol of C at 25 oC and isothermally adds B a little at a time, a two-phase system first appears when a bit more than 0.125 mol of B has been added; continuing to add B, one finds the two-phase system becomes a one-phase system when a total of 3 mol of B has been added. For a system consisting of 2.5 mol of B and 2 mol of C at 25 oC, find the number of moles of B and of C present in each phase.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 11:50, bellojamilet410
What substance has a mass of 9.5g and volume of 2.1cm^3
Answers: 2


Chemistry, 22.06.2019 19:50, nikoidurrant
What is the wavelength of a wave with a velocity of 50 m/s and a frequency of 5hz a 250 m b 0.1 m c 10m d 0.01 m
Answers: 2
Do you know the correct answer?
Liquids B and C are partially miscible at 25 oC. When one starts with 1 mol of C at 25 oC and isothe...
Questions in other subjects:



Mathematics, 24.04.2020 16:21






Arts, 24.04.2020 16:21


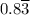 moles of A and 2.5 moles of B in the one-phase system
moles of A and 2.5 moles of B in the one-phase system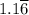 moles of A and
moles of A and 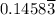 moles of B in the two-phase system
moles of B in the two-phase system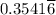 moles of B remains in the system
moles of B remains in the system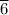 moles of A
moles of A



