
Chemistry, 18.03.2021 21:20, vanitycarraway2000
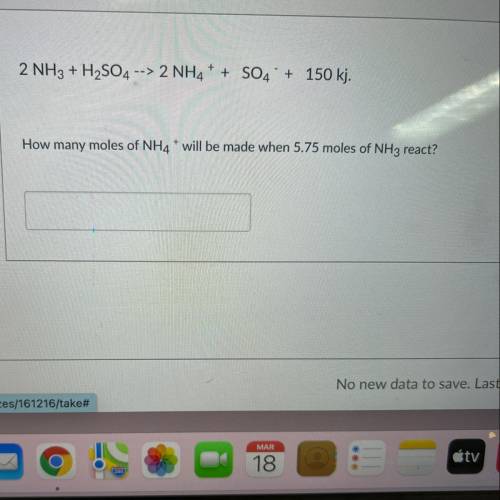
2 NH3 + H2SO4 --> 2NH4+ + SO4 + 150 kj. How many moles of NH4* will be made when 5.75 moles of NH3 react?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 06:30, rosieposie27
(1.6 × 10-19)(5.0 × 106) = c × 10d identify the missing numbers below to show the result of multiplying the numbers.
Answers: 1

Chemistry, 22.06.2019 06:30, irvinbhangal2
What effect might melting sea ice have for people who live in coastal areas?
Answers: 1

Chemistry, 22.06.2019 07:10, jasondesatnick
An experimental procedure requires a 10 ml of acid to be dissolved
Answers: 2

Chemistry, 22.06.2019 18:40, bananaslada
What is the binding energy of a nucleus that has a mass defect of 5.81*10-^29 kg a 5.23*10-^12 j b 3.15* 10^12 j c 1.57*10-3 j d 9.44*10^20 j
Answers: 1
Do you know the correct answer?
2 NH3 + H2SO4 --> 2NH4+ + SO4 + 150 kj.
How many moles of NH4* will be made when 5.75 moles of N...
Questions in other subjects:


Engineering, 19.08.2020 03:01






Mathematics, 19.08.2020 03:01


Mathematics, 19.08.2020 03:01






