
Chemistry, 18.03.2021 21:10, shukriabdisabrie
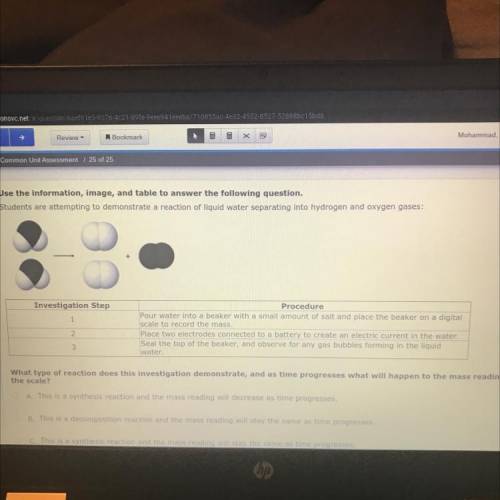
What type of reaction does this investigation demonstrate, and as time progresses what will happen to the mass reading on
the scale?
O A. This is a synthesis reaction and the mass reading will decrease as time progresses.
OB. This is a decomposition reaction and the mass reading will stay the same as time progresses,
C. This is a synthesis reaction and the mass reading will stay the same as time progresses,
D. This is a decomposition reaction and the mass reading will decrease as time progresses,

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 18:00, jeffcarpenter
Write a net ionic equation to show that hydrofluoric acid, behaves as an acid in water.
Answers: 1

Chemistry, 22.06.2019 00:00, ashleyjaslin
Which type of bonding involves the complete transfer of a valence electron from a less electrogrative atom to a more electronegative one
Answers: 1


Chemistry, 22.06.2019 10:30, Wookas8355
Great amounts of electromagnetic energy from our sun and other bodies in space travel through space. which is a logical conclusion about these electromagnetic waves? their energy must be very their frequency must be very low these waves can travel without a medium they only travel through a vacuum of space
Answers: 2
Do you know the correct answer?
What type of reaction does this investigation demonstrate, and as time progresses what will happen t...
Questions in other subjects:

SAT, 24.01.2021 22:40



Mathematics, 24.01.2021 22:40


Mathematics, 24.01.2021 22:40

Mathematics, 24.01.2021 22:40

Mathematics, 24.01.2021 22:40







