
Chemistry, 18.03.2021 20:50, aroman4511
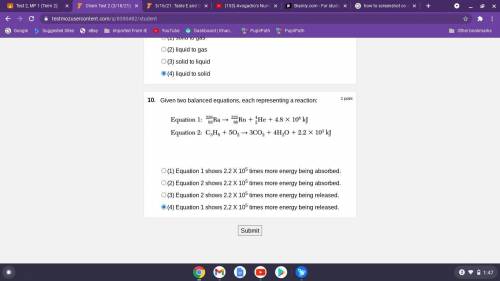
Given two balanced equations, each representing a reaction:
(1) Equation 1 shows 2.2 X 105 times more energy being absorbed. (\
2) Equation 2 shows 2.2 X 105 times more energy being absorbed.
(3) Equation 2 shows 2.2 X 105 times more energy being released.
(4) Equation 1 shows 2.2 X 105 times more energy being released.

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 08:40, kellymcdow5135
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3

Chemistry, 22.06.2019 09:20, UsedForSchool2018
Which of these statements explains the difference between nuclear binding energy and the strong nuclear force ?
Answers: 3

Chemistry, 22.06.2019 17:30, mwest200316
To find the enthalpy of a reaction in the lab, you measured the of the reactants and the change during the reaction.
Answers: 1
Do you know the correct answer?
Given two balanced equations, each representing a reaction:
(1) Equation 1 shows 2.2 X 105 times mo...
Questions in other subjects:

English, 16.03.2020 04:23


English, 16.03.2020 04:24


Mathematics, 16.03.2020 04:25


Mathematics, 16.03.2020 04:25



History, 16.03.2020 04:25






