Given 1.67 moles of K, how many moles of KBr are produced?
2 K+ 1 Br2---> 2 KBr...

Chemistry, 18.03.2021 18:20, Ariellyvz9103
Given 1.67 moles of K, how many moles of KBr are produced?
2 K+ 1 Br2---> 2 KBr

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 20.06.2019 18:02, emmamerida
If 0.414 g of hydrogen is obtained in this experiment how many grams of sulfur must be obtained
Answers: 2

Chemistry, 21.06.2019 19:00, anonymous176
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 01:50, lildestinyquintana
Ase your answer to this question on the information below. hydrocarbons and fissionable nuclei are among the sources used for the production of energy in the united states. a chemical reaction produces much less energy than a nuclear reaction per mole of reactant. the balanced chemical equation below represents the reaction of one molecule of a hydrocarbon with two molecules of oxygen. chemical equation: ch4 + 2o2 → co2 + 2h2o + 1.48 × 10−18 jthe nuclear equation below represents one of the many possible reactions for one fissionable nucleus. in this equation, x represents a missing product. nuclear equation: write an isotopic notation for the missing product represented by x in the nuclear equation.
Answers: 1

Chemistry, 22.06.2019 21:30, sullivanjakob
Isopropyl alcohol, (ch3)2choh, is a common solvent. determine the percent by mass of hydrogen in isopropyl alcohol. a) 6.71% h b) 13.4% h c) 25.0% h d) 53.3% h
Answers: 1
Do you know the correct answer?
Questions in other subjects:


Biology, 09.07.2019 06:00

Mathematics, 09.07.2019 06:00






History, 09.07.2019 06:00

Mathematics, 09.07.2019 06:00


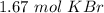
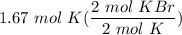 [DA] Multiply/Divide [Cancel out units]:
[DA] Multiply/Divide [Cancel out units]: 



