
Chemistry, 19.09.2019 12:10, aaminohasan142
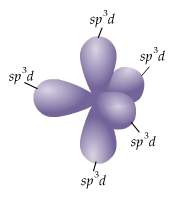
Which of the following clusters of orbitals could form a shape similar to that shown here (figure 3) in the valence shell of an isolated atom or one about to enter into bonding with other atoms?
a) five sp³d
b) three sp² and one p orbital

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 02:30, brittanysanders
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2


Chemistry, 22.06.2019 19:30, amandamiro05
Helium decays to form lithium. which equation correctly describes this decay?
Answers: 2

Chemistry, 22.06.2019 20:20, carcon2019
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3
Do you know the correct answer?
Which of the following clusters of orbitals could form a shape similar to that shown here (figure 3)...
Questions in other subjects:

Mathematics, 30.05.2020 21:57

Mathematics, 30.05.2020 21:57



History, 30.05.2020 21:57

English, 30.05.2020 21:57


Mathematics, 30.05.2020 21:57


Geography, 30.05.2020 21:57






