
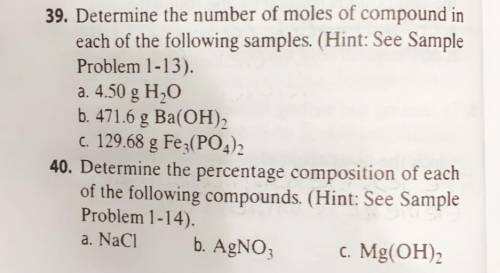
39. Determine the number of moles of compound in
each of the following samples. (Hint: See Sample
Problem 1-13).
a. 4.50 g H2O
b. 471.6 g Ba(OH)2
C. 129.68 g Fe3(PO4)2
40. Determine the percentage composition of each
of the following compounds. (Hint: See Sample
Problem 1-14).
b. AgNO3
.c. Mg(OH)2
a. NaCl
Help me please for this two question

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 06:00, girly37
In 1901, thomas edison invented the nickel-iron battery. the following reaction takes place in the battery. fe(s) + 2 nio(oh)(s) + 2 h2o(l) fe(oh)2(s) + 2 ni(oh)2(aq) how many mole of fe(oh)2, is produced when 5.35 mol fe and 7.65 mol nio(oh) react?
Answers: 3

Chemistry, 22.06.2019 06:30, caitybugking
Type the correct answer in the box. spell all words correctly. what is the correct term for living the most sustainable life you can within your current circumstances? when your are being as sustainable as you can within your current lifestyle, you are said to be sustainability.
Answers: 3

Chemistry, 22.06.2019 12:10, yootmytoot
Achemistry student needs to standardize a fresh solution of sodium hydroxide. he carefully weighs out of oxalic acid , a diprotic acid that can be purchased inexpensively in high purity, and dissolves it in of distilled water. the student then titrates the oxalic acid solution with his sodium hydroxide solution. when the titration reaches the equivalence point, the student finds he has used of sodium hydroxide solution. calculate the molarity of the student's sodium hydroxide solution. be sure your answer has the correct number of significant digits.
Answers: 1
Do you know the correct answer?
39. Determine the number of moles of compound in
each of the following samples. (Hint: See Sample
Questions in other subjects:

Geography, 23.07.2019 06:30


Mathematics, 23.07.2019 06:30

History, 23.07.2019 06:30

Biology, 23.07.2019 06:30

Mathematics, 23.07.2019 06:30










