
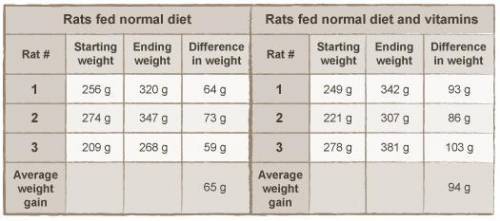
A scientist was asked to test the effect of a new vitamin for rats. His hypothesis was that young rats that had vitamins added to their food would gain weight faster than young rats fed a normal diet. He predicted that if young rats were fed vitamins, then they would gain more weight by the end of the experiment. He tested the effect of adding vitamins to some of the rats' diet by measuring the amount of weight each rat gained after three months. He then determined the average weight gained in each group. To analyze his results, he put the data he collected into the table shown below.
How was or wasn't the scientific method followed in this experiment?
A. The scientific method was followed because there was an experimental group and a control group.
B. The scientific method was not followed because the experiment was not repeated many times by many different people.
C. The scientific method was not followed because the scientist did not write down the question to be tested.
D. The scientific method was followed because the experiment tested the hypothesis and produced reliable results.

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 21:20, 50057543
Phosgene (carbonyl chloride), cocl2, is an extremely toxic gas that is used in manufacturing certain dyes and plastics. phosgene can be produced by reacting carbon monoxide and chlorine gas at high temperatures: co(g) cl2(g)⇌cocl2(g) carbon monoxide and chlorine gas are allowed to react in a sealed vessel at 477 ∘c . at equilibrium, the concentrations were measured and the following results obtained: gas partial pressure (atm) co 0.830 cl2 1.30 cocl2 0.220 what is the equilibrium constant, kp, of this reaction
Answers: 2

Chemistry, 22.06.2019 22:30, gonzalesalexiaouv1bg
What if it is did darwin used to support his theory of evolution
Answers: 1

Chemistry, 22.06.2019 23:30, znewkirk4741
Imagine a small synthetic vesicle made from pure phospholipids enclosing an interior lumen containing 1 mm glucose and 1 mm sodium chloride. if the vesicle is placed in pure water, which of the following happens faster? a. na+ diffuses out. b. cl– diffuses out. c. h2o diffuses in. d. glucose diffuses out. e. sodium chloride diffuses out.
Answers: 3

Chemistry, 23.06.2019 05:00, rosezgomez97
Asolution is made by dissolving 2.3 moles of sodium chloride (nacl) in 0.155 kilograms of water. if the molal boiling point constant for water (kb) is 0.51 °c/m, what would be the boiling point of this solution? show all the steps taken to solve this problem.
Answers: 1
Do you know the correct answer?
A scientist was asked to test the effect of a new vitamin for rats. His hypothesis was that young ra...
Questions in other subjects:


Mathematics, 21.06.2021 04:40




History, 21.06.2021 04:40

Mathematics, 21.06.2021 04:40



Mathematics, 21.06.2021 04:40






