
Chemistry, 18.03.2021 09:10, xelynncaldera
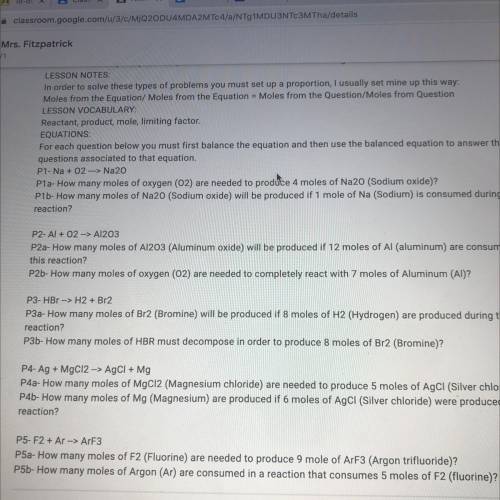
P5-F2 + Ar --> ArF3
P5a- How many moles of F2 (Fluorine) are needed to produce 9 mole of ArF3 (Argon trifluoride)?
P5b- How many moles of Argon (Ar) are consumed in a reaction that consumes 5 moles of F2 (fluorine)?
I need the answers for the p5 part please help

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 09:30, kevinh2683
Apump contains 0.5 l of air at 203 kpa. you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 16:30, montanolumpuy
How many moles of sulfuric acid (h2so4) are needed to react completely with 6.8 moles of lithium hydroxide (lioh)? 2lioh + h2so4 → li2so4 + 2h2o a. 3.4 mol h2so4b. 6.8 mol h2so4 c. 10.2 mol h2so4 d. 13.6 mol h2so4
Answers: 3

Do you know the correct answer?
P5-F2 + Ar --> ArF3
P5a- How many moles of F2 (Fluorine) are needed to produce 9 mole of ArF3 (A...
Questions in other subjects:




Mathematics, 24.11.2019 00:31



Mathematics, 24.11.2019 00:31



History, 24.11.2019 00:31






