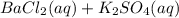Chemistry, 18.03.2021 02:50, Gimagg5549
What are the products from the following double - replacement reaction ? BaCl 2(aq) +K 2 SO 4(aq) a . BaSO 3 and KCI b. and KClO 4 c. BaSO 3 KClO 4 d. BaSO 4 and KClO 4 e . BaSO 4 and KCI

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, cutebab4786
Consider the point on the plot where 10.0 g of naoh have been added. what amount of naoh, in moles, has been added? 0.308 mol fecl3 initially present
Answers: 1

Chemistry, 22.06.2019 04:50, psychocatgirl1
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 11:30, elijah1090
Aperfume bottle is dropped in the corner of a room. the odor of the perfume can be detected on the other side of the room. which statement best describes this observation?
Answers: 2
Do you know the correct answer?
What are the products from the following double - replacement reaction ? BaCl 2(aq) +K 2 SO 4(aq) a...
Questions in other subjects:





Health, 04.02.2020 08:44

History, 04.02.2020 08:44

Business, 04.02.2020 08:44

History, 04.02.2020 08:44

Mathematics, 04.02.2020 08:44

Computers and Technology, 04.02.2020 08:44