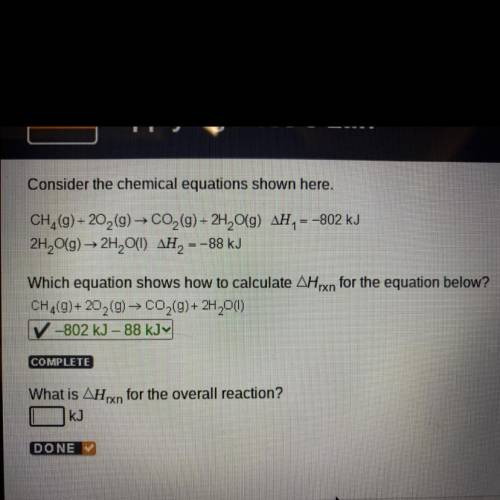
Consider the chemical equations shown here.
CH (9) +20,(9) → CO2 (9)+ 2H, O(9) AH, = -802 kJ
...

Chemistry, 18.03.2021 02:40, graceduke2005p6z8yp
Consider the chemical equations shown here.
CH (9) +20,(9) → CO2 (9)+ 2H, O(9) AH, = -802 kJ
2H2O(g) → 2H, O(1) AH, -
= -88 kJ
Which equation shows how to calculate Arxn for the equation below?
CH,(9) + 20 (0) - CO,(9)+ 2H 0(1)
-802 kJ - 88 KJY
COMPLETE
What is AHxn for the overall reaction?
KJ
PLEASE FOCUS ON THE SECOND PART !!
Help

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, kingbot350
How many grams of magnesium metal will react completely with 8.3 liters of 5.5m hcl? show all work
Answers: 1

Chemistry, 23.06.2019 03:00, yoongislaugh
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

Chemistry, 23.06.2019 03:30, memester74
Scientists often deal with numbers that are either very large or very small. for example, the radius of the sun is approximately 696,000 kilometers, while bacterial cells are as small as 1.9 × 10-4 millimeters. express each of these numbers in an alternate form.
Answers: 3

Chemistry, 23.06.2019 08:00, george27212
What is the temperature in kelvin of a gas if it is allowed to expand from 1.50 l to 4.50 l? the initial temperature is 10.0°c and pressure is constant throughout the change. which equation should you use? t2= v2/v1 t1 what is the final temperature? ⇒ 849 k these are the answers.
Answers: 1
Do you know the correct answer?
Questions in other subjects:




Biology, 01.09.2019 03:30






Mathematics, 01.09.2019 03:30






