
Chemistry, 18.03.2021 02:10, krystlemiller11211
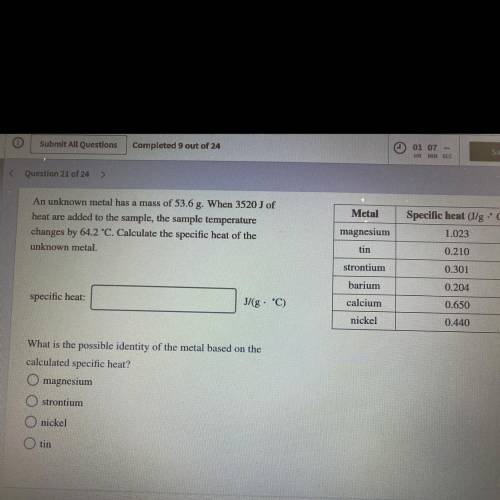
An unknown metal has a mass of 53.6 g. When 3520J of heat are added to the sample, the sample temp. changes by 64.2°C. Calculate specific heat of the unknown metal. what is the metal?

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:10, goodygoodgirlygirl
Which of the following best describes the formation of plasma?
Answers: 1


Do you know the correct answer?
An unknown metal has a mass of 53.6 g. When 3520J of heat are added to the sample, the sample temp....
Questions in other subjects:

Mathematics, 29.07.2021 20:50






Spanish, 29.07.2021 20:50








