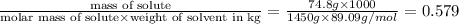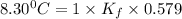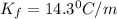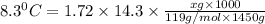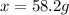Chemistry, 18.03.2021 01:20, Drevei6969
When 74.8g of alanine C3H7NO2 are dissolved in 1450.g of a certain mystery liquid X, the freezing point of the solution is 8.30°C less than the freezing point of pure X. Calculate the mass of potassium bromide that must be dissolved in the same mass of X to produce the same depression in freezing point. The van't Hoff factor =i1.72 for potassium bromide in X.

Answers: 1
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 02:00, lwattsstudent
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al
Answers: 1

Chemistry, 22.06.2019 03:30, tbeck225
If a solution is considered basic, then a) the hydroxide ion and hydronium ion concentrations are equal. b) the hydroxide ion concentration is less than the hydronium ion concentration. c) the hydronium ion concentration is greater than the hydroxide ion concentration. d) the hydroxide ion concentration is greater than the hydronium ion concentration.
Answers: 1

Chemistry, 22.06.2019 14:00, luisaareli6298
Calculate the energy required to ionize a hydrogen atom to an excited state where the electron is initially in the n = 5 energy level. report your answer in kilojoules
Answers: 1
Do you know the correct answer?
When 74.8g of alanine C3H7NO2 are dissolved in 1450.g of a certain mystery liquid X, the freezing po...
Questions in other subjects:


English, 29.01.2021 20:40

Mathematics, 29.01.2021 20:40




History, 29.01.2021 20:40

Health, 29.01.2021 20:40


Mathematics, 29.01.2021 20:40


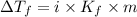
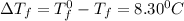 = Depression in freezing point
= Depression in freezing point
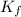 = freezing point constant =
= freezing point constant =