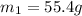Chemistry, 18.03.2021 01:10, katherineweightman
A sample of aluminum, which has a specific heat capacity of 0.897·J·g−1°C^−1 , is put into a calorimeter (see sketch at right) that contains 300.0g of water. The aluminum sample starts off at 94.5°C and the temperature of the water starts off at 21.0°C .When the temperature of the water stops changing it's 23.8°C .The pressure remains constant at 1atm .Calculate the mass of the aluminum sample.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 12:30, fvmousdiana
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3

Chemistry, 22.06.2019 13:30, bryce99
In a ni-cd battery, a fully charged cell is composed of nickelic hydroxide. nickel is an element that has multiple oxidation states. assume the following proportions of the states: nickel charge proportions found 0 0.17 +2 0.3 +3 0.33 +4 0.5 (a) determine the mean of the nickel charge. enter the answer to 2 decimal places.(b) determine the cumulative distribution function of nickel charge.
Answers: 2

Chemistry, 22.06.2019 15:00, MilanPatel
How is the shape of the poem “peer” connected to its meaning?
Answers: 2
Do you know the correct answer?
A sample of aluminum, which has a specific heat capacity of 0.897·J·g−1°C^−1 , is put into a calorim...
Questions in other subjects:


Biology, 16.09.2019 23:30


Biology, 16.09.2019 23:30




Mathematics, 16.09.2019 23:30

Mathematics, 16.09.2019 23:30

Mathematics, 16.09.2019 23:30


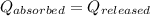
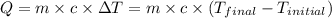
![m_1\times c\times (T_{final}-T_1)=-[m_2\times c\times (T_{final}-T_2)]](/tpl/images/1195/8097/b0e58.png)
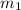 = mass of aluminium = ?
= mass of aluminium = ?
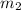 = mass of water = 300.0 g
= mass of water = 300.0 g
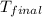 = final temperature =
= final temperature = 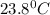
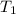 = temperature of aluminium =
= temperature of aluminium = 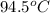
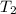 = temperature of water =
= temperature of water = 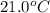
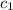 = specific heat of aluminium =
= specific heat of aluminium = 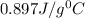
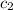 = specific heat of water =
= specific heat of water = 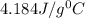
![-[m_1\times c_1\times (T_{final}-T_1)]=[m_2\times c_2\times (T_{final}-T_2)]](/tpl/images/1195/8097/92a72.png)
![-[m_1\times 0.897\times (23.8-94.5)^0C]=[300.0g\times 4.184\times (23.8-21.0)]](/tpl/images/1195/8097/6f259.png)