
Chemistry, 17.03.2021 23:50, JamesLachoneus
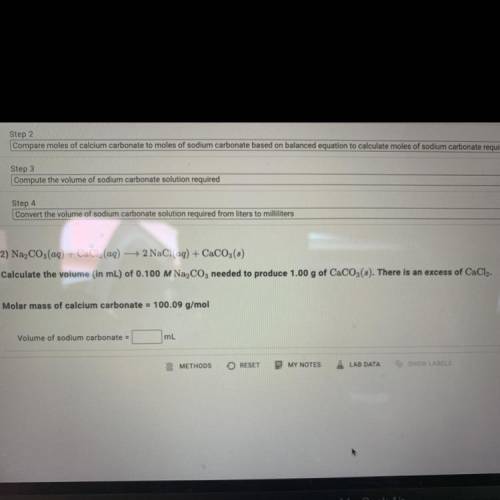
Calculate the volume (in mL) of 0.100 M Na, C03 needed to produce 1.00 g of CaCO3(s).
There is an excess of CaCl2.
Molar mass of calcium carbonate = 100.09 g/mol
Volume of sodium carbonate = ?mL

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:00, SmolBeanPotato
What is the volume of the fluid in the graduated cylinder with accuracy and measured to the correct degree of precision? 41.2 ml 42.0 ml 41.23 ml 41.89 ml
Answers: 1

Chemistry, 22.06.2019 07:00, mayamabjishovrvq9
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1


Chemistry, 22.06.2019 13:00, devontemiles8868
One of the hopes for solving the world's energy problem is to make use of the fusion reaction 21h +31h --> 42he + 10n + energy how much energy is released when 1 mol of deuterium is fused with 1 mol of tritium according to the above reaction? the masses of the atoms and the neutrons are as follows: 21h = 2.0140 amu 31h = 3.01605 amu 42he = 4.002603 amu 10n = 1.008665 amu. the speed of light is 2.9979 x 108 m/s.
Answers: 1
Do you know the correct answer?
Calculate the volume (in mL) of 0.100 M Na, C03 needed to produce 1.00 g of CaCO3(s).
There is an e...
Questions in other subjects:



Social Studies, 07.08.2021 08:30

Spanish, 07.08.2021 08:30












