STEP 5: LEAD
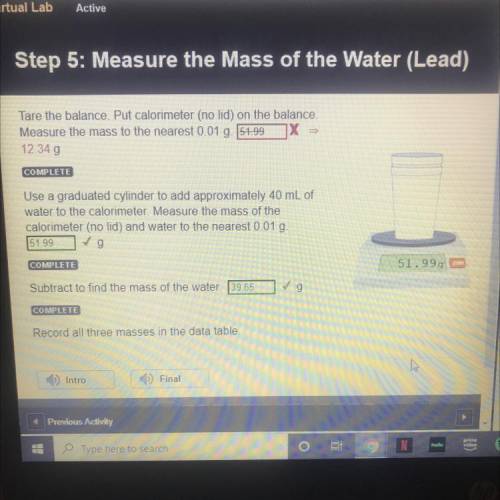
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to t...

Chemistry, 17.03.2021 23:50, bartekpiglo
STEP 5: LEAD
Tare the balance. Put calorimeter (no lid) on the balance.
Measure the mass to the nearest 0.01 g.
12.34 g
Use a graduated cylinder to add approximately 40 mL of
water to the calorimeter. Measure the mass of the
calorimeter (no lid) and water to the nearest 0.01 g.
51.99 g
Subtract to find the mass of the water. 39.65 g

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 18:40, johnnysteeler9934
What is one real world example of a colligative property?
Answers: 2

Chemistry, 22.06.2019 20:00, 20calzoy
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: cac2 (s) + 2h2o (g) → c2h2 (g) + caoh2 (s) =δh−414.kj in the second step, acetylene, carbon dioxide and water react to form acrylic acid: 6c2h2 (g) + 3co2 (g) + 4h2o (g) → 5ch2chco2h (g) =δh132.kj calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest kj .
Answers: 3

Chemistry, 22.06.2019 23:30, sweaversw9602
What are the similarities between compounds and mixtures?
Answers: 3
Do you know the correct answer?
Questions in other subjects:








Computers and Technology, 06.08.2019 04:20








