
Chemistry, 17.03.2021 23:50, bella122805
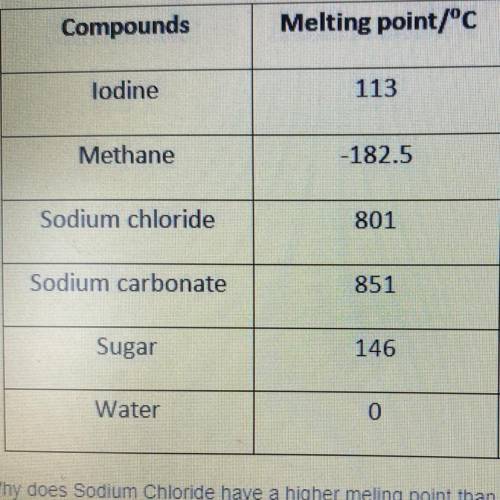
Why does Sodium Chloride have a higher melting point than Sugar?
a. intermolecular forces are weaker
b. intermolecular forces are slightly stronger
c. intermolecular forces are very strong
d. melting point is based on composition and not bonding

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:10, mistiehaas
Which of the following elements would you expect to have the lowest ionization energy value? fluorine, lithium, neon, nitrogen
Answers: 2

Chemistry, 22.06.2019 10:20, blondielocks2002
Gwhich r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? which r group would most likely be found in a hydrophobic area of the tertiary structure of a globular protein? −ch2−oh −ch2−o||c−nh2 −ch2−coo− −ch2−ch2−ch2−ch2−n+h3
Answers: 3


Chemistry, 22.06.2019 22:00, shaylasimonds587
The volume of an unknown substance in a sealed glass jar is 50 milliliters. the volume of the jar is 200 milliliters. which state of matter could the substance be?
Answers: 2
Do you know the correct answer?
Why does Sodium Chloride have a higher melting point than Sugar?
a. intermolecular forces are weake...
Questions in other subjects:


History, 27.06.2019 16:30




Chemistry, 27.06.2019 16:30

Mathematics, 27.06.2019 16:30


Geography, 27.06.2019 16:30






