
Chemistry, 17.03.2021 23:40, nataliecooper542
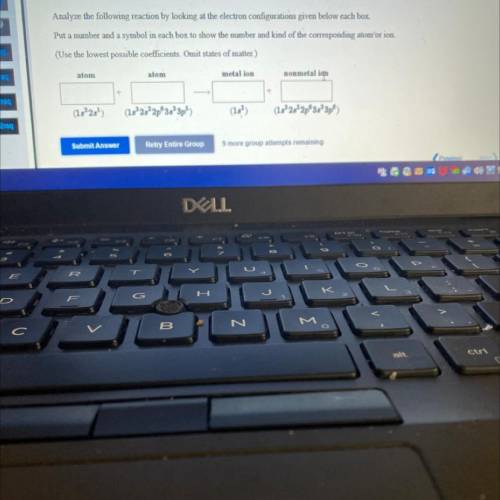
Analyze the following reaction by looking at the electron configurations given below each box.
pts
Put a number and a symbol in each box to show the number and kind of the corresponding atomʻor ion.
pts 2reg
(Use the lowest possible coefficients. Omit states of matter.)
pts 2req
atom
atom
metal ion
nonmetal ion
pts 2reg
(182)
(1s 2s 2p 3s 3p5)
(15)
(15°25² 2p 3s 3p
1 pts 2rea

Answers: 3
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 12:30, americanbellabeauty
Acontrol during an experiment. might change remains constant does not exist does change
Answers: 1

Do you know the correct answer?
Analyze the following reaction by looking at the electron configurations given below each box.
pts<...
Questions in other subjects:


Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00

Mathematics, 05.05.2021 01:00

English, 05.05.2021 01:00


Business, 05.05.2021 01:00



Biology, 05.05.2021 01:00






