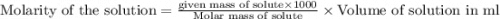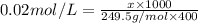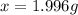Please if you know help
the formula of copper sulfate crystals is CuSo4.5H2O. 400ml of 0.02mol/l copper (ii) sulfate solution is needed to be prepered .
a. calculate the molar mass of the solute .
b. calculate the mass of the solute that sould be weighted to prepare this solution .
c. describe in details the steps of preperation of the solution

Answers: 1
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:00, anonymous176
Iknow the answer to 13 is b and 14 is d. i just need to know why the correct answers are correct
Answers: 3

Chemistry, 22.06.2019 13:00, nauticatyson9
Jose and eric were given four samples in lab. the results of their analysis are shown in the table. based on the data they collected, which sample is most likely a metal?
Answers: 1

Chemistry, 22.06.2019 21:30, starl0rd211
Describe at least two advantages and two disadvantages of using hydropower as a source of energy.
Answers: 2

Chemistry, 22.06.2019 22:30, StupidFatChipmunk
What must be in balance for temperatures to remain constant?
Answers: 1
Do you know the correct answer?
Please if you know help
the formula of copper sulfate crystals is CuSo4.5H2O. 400ml of 0.02mol/l co...
Questions in other subjects:

Spanish, 28.05.2021 05:50

Chemistry, 28.05.2021 05:50

History, 28.05.2021 05:50

Mathematics, 28.05.2021 05:50

Health, 28.05.2021 05:50

Spanish, 28.05.2021 05:50

Social Studies, 28.05.2021 05:50



Mathematics, 28.05.2021 05:50


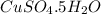 and dissolve in water until the volume is 400 ml
and dissolve in water until the volume is 400 ml