Hydrobromic acid dissolves solid iron according to the
following reaction:
Fe(s) + 2 HBr(aq)...

Chemistry, 13.03.2021 08:10, moranmar2283
Hydrobromic acid dissolves solid iron according to the
following reaction:
Fe(s) + 2 HBr(aq) → FeBr2(aq) + H2(9)
Part A
What mass of HBr (in g) would you need to dissolve a 3.2 - g pure iron
Express your answer using two significant figures.
Part B
What mass of H, would be produced by the complete reaction of the iron

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 01:00, angelteddy033
Which of the following is not a true statement about dwarf planets? a the kuiper belt contains comets, asteroids, and dwarf planets. b ceres is a dwarf planet located in the kuiper belt. c the largest known dwarf planet in the solar system is named eris.
Answers: 2

Chemistry, 22.06.2019 04:30, falishaduncanovmtz2
Electrons are extremely important to what area of technology? a) anti-aging research b) household product development c) electronics d) drug discovery
Answers: 3

Chemistry, 22.06.2019 11:30, chelseychew32
What is the main reason why some developing countries fear the increase the free trade policies around the world?
Answers: 2

Chemistry, 23.06.2019 07:50, alexusnicole817
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
Do you know the correct answer?
Questions in other subjects:

Mathematics, 03.07.2019 17:00

Mathematics, 03.07.2019 17:00


History, 03.07.2019 17:00





Mathematics, 03.07.2019 17:00

Mathematics, 03.07.2019 17:00


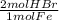 = 0.115 mol HBr
= 0.115 mol HBr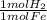 = 0.0573 mol H₂
= 0.0573 mol H₂



