
Chemistry, 13.03.2021 04:50, genyjoannerubiera
Text 8.7 Using the virial equation of state for hydrogen at 298 K given in problem 7 (text 8.6), calculate a. The fugacity of hydrogen at 500 atm and 298 K b. The pressure at which they fugacity is twice the pressure c. The change in Gibbs free energy caused by a compression of 1 mole of hydrogen from 1 to 500 atm. What is the magnitude of the contribution to (c) caused by the non ideality of hydrogen

Answers: 2
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 19:30, mazielynn84
How many molecules of sucrose c12h22o11 are there in 454 grams of sucrose
Answers: 1

Chemistry, 21.06.2019 23:00, orlando19882000
Ahypothrticalax type of ceramic material is known to have a density of 2.10 g/cm3 and a unit cell of cubic symmetry with a cell edge length of 0.57 nm. the atomic weights of the a and x elements are 28.5and 30.0 g/mol, respectively. on the basis of this information, which of the following crystal structures is (are) possible for this material: sodium chloride, cesium chloride, or zinc blende
Answers: 1

Chemistry, 22.06.2019 07:30, nayiiii1874
What three things determine the shape and size of a puddle when water is poured out onto a surface
Answers: 2

Chemistry, 22.06.2019 12:40, whitethunder05
When 13.3 g koh is dissolved in 102.7 g of water in a coffee-cup calorimeter, the temperature rises from 21.4 °c to 31.53 °c. what is the enthalpy change per gram of koh (j/g) dissolved in the water? * take the density of water as 1.00 g/ml. * assume that the solution has a specific heat capacity of 4.18 j/g*k. enter to 1 decimal place. do not forget the appropriate sign /(+). canvas may auto-delete the (+) sign
Answers: 2
Do you know the correct answer?
Text 8.7 Using the virial equation of state for hydrogen at 298 K given in problem 7 (text 8.6), cal...
Questions in other subjects:




Mathematics, 24.01.2020 22:31

Mathematics, 24.01.2020 22:31

Mathematics, 24.01.2020 22:31

Mathematics, 24.01.2020 22:31




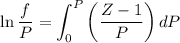
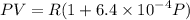 ........(1)
........(1)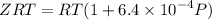
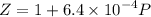
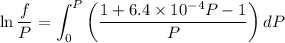
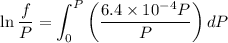
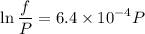
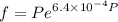
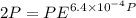
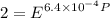
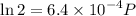
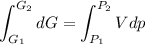
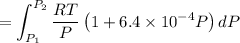
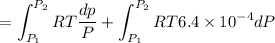
![$\Delta G=R[\ln\frac{P_2}{P_1}+6.4 \times 10^{-4}(P_2-P_1)]$](/tpl/images/1193/5881/be85b.png)
![$\Delta G=8.314\times 298[\ln\frac{500}{1}+6.4 \times 10^{-4}(500-1)]$](/tpl/images/1193/5881/16a41.png)





