
Chemistry, 13.03.2021 04:00, youngcie04
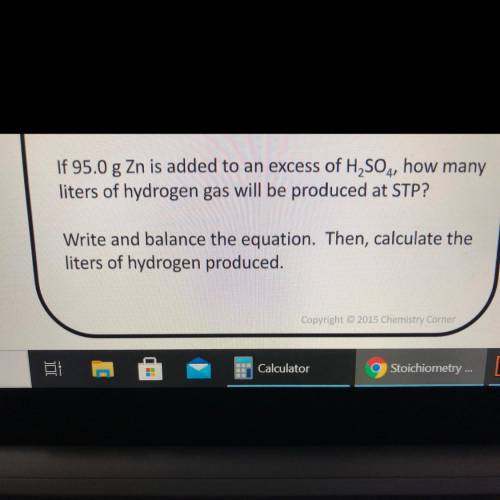
If 95.0 g Zn is added to an excess of H2SO4, how many
liters of hydrogen gas will be produced at STP?
Write and balance the equation. Then, calculate the
liters of hydrogen produced.
Please help

Answers: 2
Other questions on the subject: Chemistry


Chemistry, 22.06.2019 16:00, anferneebcoleman
How many moles of oxygen react with 12 moles of aluminum
Answers: 1

Chemistry, 22.06.2019 17:00, princessakosua2
The msds for glacial acetic acid says that it is a flammable liquid that can severely burn any human tissue it comes in contact with. it reacts with bases, various metals, and strong oxidizing agents. its vapors can form explosive mixtures with air.
Answers: 1

Chemistry, 22.06.2019 18:00, darrell1168
How does climate change cause the ocean's thermohaline current to slow down?
Answers: 3
Do you know the correct answer?
If 95.0 g Zn is added to an excess of H2SO4, how many
liters of hydrogen gas will be produced at ST...
Questions in other subjects:




History, 19.12.2020 14:00



Mathematics, 19.12.2020 14:00

Mathematics, 19.12.2020 14:00








