Part E
In part E, you'll use your answers from parts B, C, and D to find the
average density...

Chemistry, 13.03.2021 02:40, hickslily9
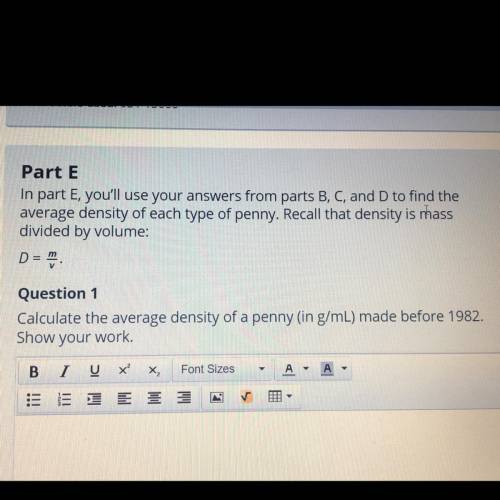
Part E
In part E, you'll use your answers from parts B, C, and D to find the
average density of each type of penny. Recall that density is mass
divided by volume:
D =
Question 1
Calculate the average density of a penny (in g/mL) made before 1982.
Show your work.

Answers: 3
Other questions on the subject: Chemistry

Chemistry, 21.06.2019 23:30, hellokitty1647
For the following dehydrohalogenation (e2) reaction, draw the zaitsev product(s) resulting from elimination involving c3–c4 (i. e., the carbon atoms depicted with stereobonds). show the product stereochemistry clearly. if there is more than one organic product, both products may be drawn in the same box. ignore elimination involving c3 or c4 and any carbon atom other than c4 or c3.
Answers: 3

Chemistry, 22.06.2019 21:30, emmalucilleblaha1995
Achemical reaction is done in the setup shown, resulting in a change of mass. what will happen if the same reaction is done in a sealed container that is placed on the electronic balance?
Answers: 1

Chemistry, 23.06.2019 02:00, xbeatdroperzx
Which statement is true about the model of the electromagnetic spectrum a: it change the frequencies of light. b: it compare wavelengths of light. c: the color of light waves can be changed using the model. d: the intensities of light waves can be decreased using the model.
Answers: 2

Chemistry, 23.06.2019 06:30, amylumey2005
The polarity of an oxygen-hydrogen bond is higher than the polarity of a nitrogen-hydrogen bond, allowing amines to be more soluble than alcohols.
Answers: 3
Do you know the correct answer?
Questions in other subjects:








History, 24.08.2020 14:01


Advanced Placement (AP), 24.08.2020 14:01