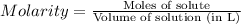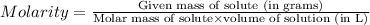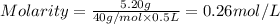Answers: 1
Other questions on the subject: Chemistry

Chemistry, 22.06.2019 00:30, tdowling331
What must happen before a body cell can begin mitotic cell division
Answers: 2

Chemistry, 22.06.2019 06:00, lindseyklewis1p56uvi
Ethanol (c2h5oh) is produced from the fermentation of sucrose in the presence of enzymes. c12h22o11(aq) + h2o(g) 4 c2h5oh(l) + 4 co2(g) determine the theoretical yield and the percent yields of ethanol if 680. g sucrose undergoes fermentation and 326.5 g ethanol is obtained. theoretical _ g _ percent %
Answers: 1

Chemistry, 22.06.2019 13:50, aesthetickait
How does the motion of particles in a gas change as the gas cools
Answers: 2

Chemistry, 22.06.2019 15:10, strodersage
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
Do you know the correct answer?
The molar mass of naoh is 40.00 g/mol. if 5.20 g naoh are dissolved in 0.500 l of solution, what is...
Questions in other subjects:



Mathematics, 28.01.2020 05:31




Mathematics, 28.01.2020 05:31

Biology, 28.01.2020 05:31

Mathematics, 28.01.2020 05:31

Health, 28.01.2020 05:31